PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release St. Paul, MN – October…
PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release
St. Paul, MN – October 18, 2023. Cardio Flow, Inc., a privately held medical device company and developer of minimally invasive devices to treat peripheral artery disease (PAD), today announced it recently received U.S. Food and Drug Administration (FDA) 510(k) clearance for the company’s FreedomFlow Orbital Atherectomy Peripheral Platform.
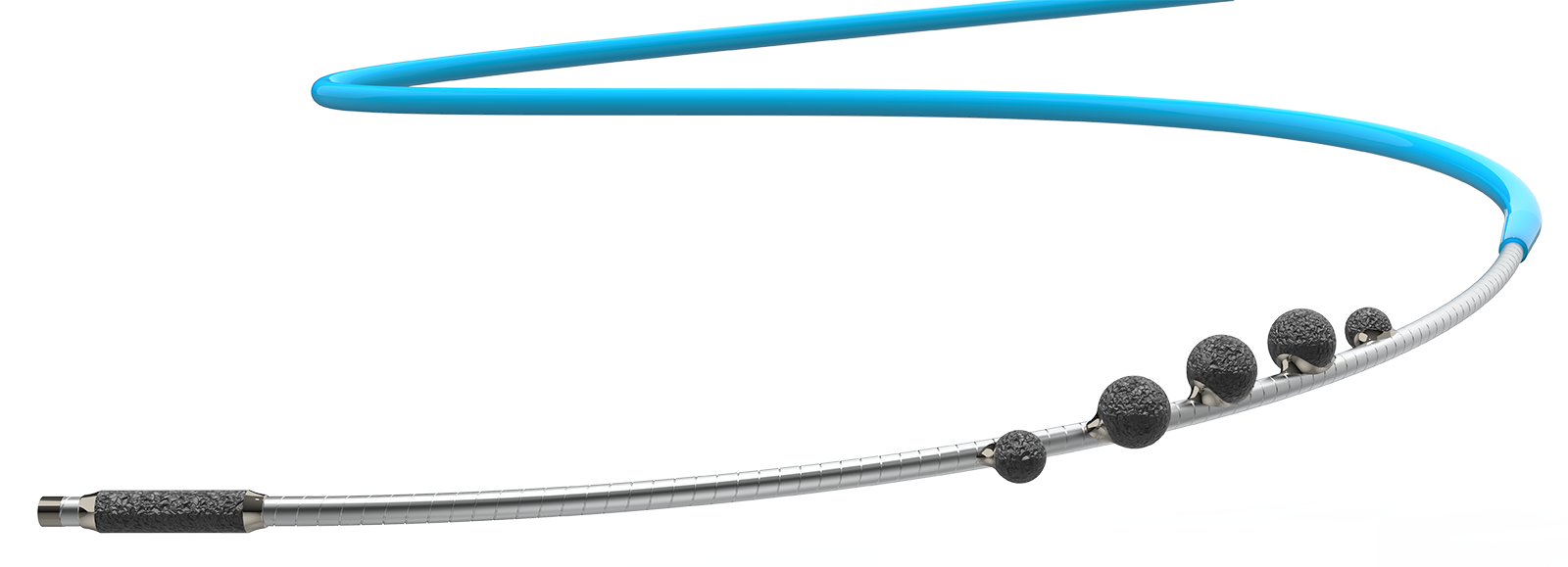
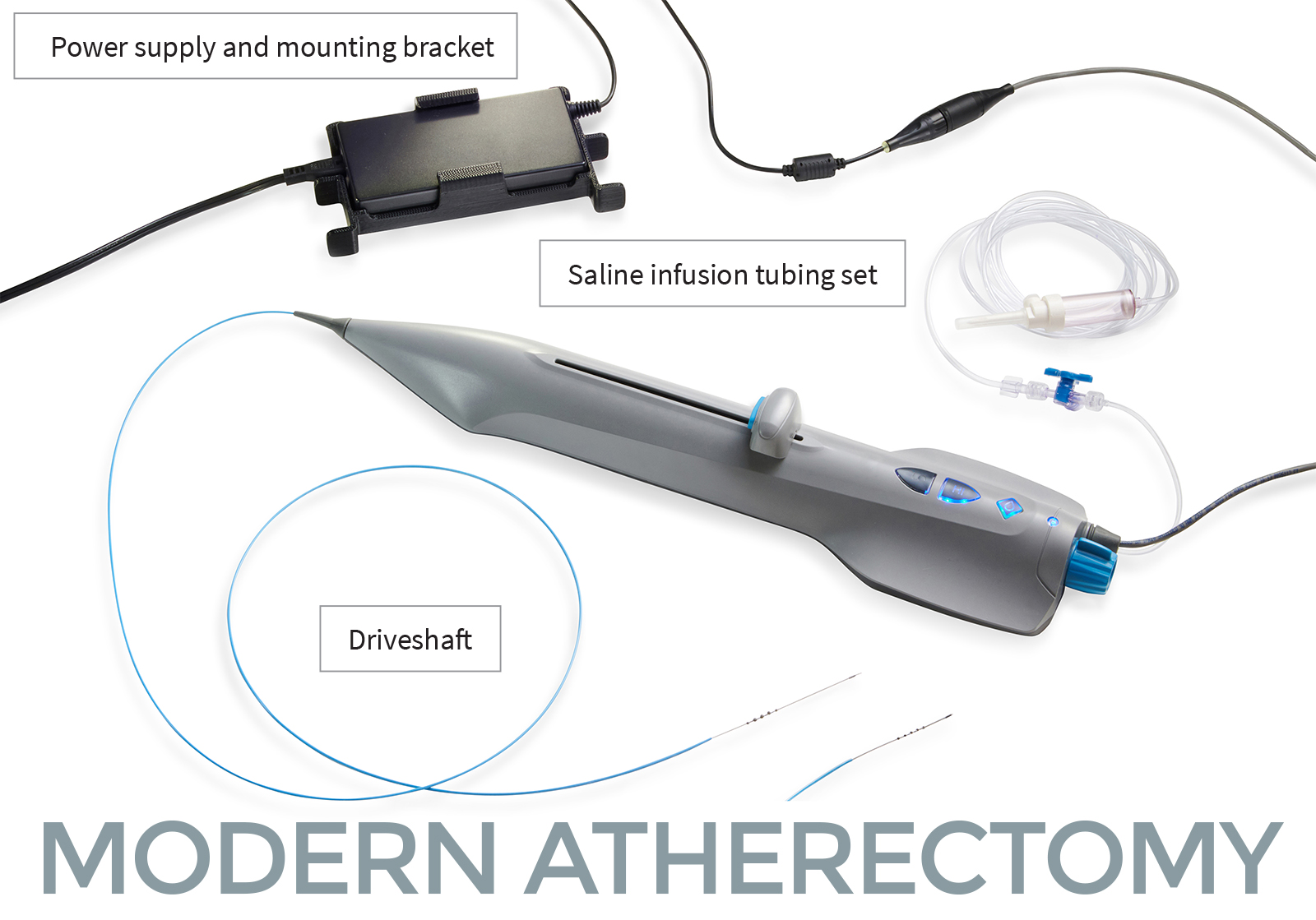
The FreedomFlow platform is designed with a modern mechanism of action to clear plaque blockages in the arteries of the legs. This proprietary, catheter-based design leverages the physics of angular momentum, creating a spiral geometry that puts five diamond-coated spheres in simultaneous contact with the vessel wall, whether advancing or retracting. A diamond-coated tip also helps ease the driveshaft through tight blockages.
This unique approach gives physicians a highly efficient, effective, and flexible way to treat complex PAD in a wide range of vessel diameters—from 2 mm in the ankle to 8 mm in the hip—and greater versatility in treating multiple arteries and multiple blockages in the same vessel, all with a single device.
The simplicity of the FreedomFlow platform likewise offers time savings and cost efficiencies, with quick and simple device setup, no capital equipment, no lubricant, and reduced inventory needs. It also provides faster run times, consistent treatment outcomes, and a nearly flat learning curve for physicians, making it an ideal choice for today’s hospitals, ambulatory surgical centers, and office-based labs.
Dr. Thomas Davis, M.D., the Director of Cardiovascular Research at Ascension St. John’s Hospital in Detroit, MI, and a recognized leader in the treatment of PAD and critical limb-threatening ischemia, commented:
“The incidence of patients presenting with multi-level PAD has increased dramatically in my practice, so having a flexible, efficient, and easy-to-use device that can treat PAD in a broad range of vessel sizes is a key advantage for physicians and patients as we seek to reduce the number of PAD-related amputations.”
Michael J. Kallok, Ph.D., CEO of Cardio Flow, stated:
“Cardio Flow is committed to providing meaningful solutions that directly address the needs of physicians and their PAD patients through innovative product development. Many of the existing atherectomy devices on the market have various design constraints and capital equipment costs.
With FreedomFlow, we strove to provide physicians with the freedom to treat complex PAD hip to heel with a simple yet sophisticated device that would answer the call for flexible treatment options and cost savings for healthcare systems. We’re excited that FreedomFlow will now be available to physicians.”
About Cardio Flow, Inc.
Cardio Flow, Inc., is a privately held medical device company located in St. Paul, MN, which designs and develops minimally invasive peripheral vascular products with the goal of providing physicians with better treatment options for peripheral vascular disease to improve patients’ lives.
See cardioflow.net for more information.
About PAD
Peripheral Arterial Disease is a chronic circulatory condition that, if left untreated, can result in unnecessary limb amputations. In the United States, it’s estimated that 8 to 10 million individuals suffer from PAD annually. Critical Limb-Threatening Ischemia (CLTI), a more severe form of PAD, currently affects approximately 2 to 3 million people in the U.S. and is expected to exceed 4 million by 2030.
Rates of amputation have steadily been increasing in the U.S. over the past 20 years. In fact, the Amputation Reduction and Compassion (ARC) Act, introduced in Congress in June 2023, seeks to reduce the high rates of amputation in the U.S. via professional awareness, patient empowerment, and early diagnosis and care.
Sources: Mark A. Creager, et. al., “Reducing Nontraumatic Lower-Extremity Amputations by 20% by 2030: Time to get to Our Feet: A Policy Statement from the American Heart Association,” Circulation, 143:17, April 27, 2021; American Diabetes Association press release, “ADA Unveils Amputation Prevention Alliance to Address the Diabetes-Related Amputation Pandemic,” September 22, 2022; Mary L. Yost, “Critical Limb Ischemia: Volume 1. United States Epidemiology, 2016 Supplement,” The Sage Group, 2017; Jihad A. Mustapha, et. al., “Treatment of Peripheral Artery Disease and Critical Limb Ischemia: An Observational Michigan Medical Analysis,” Journal of Critical Limb Ischemia, 3:2, June 2023; Amputation Reduction and Compassion Act of 2023, H.R. 4216, 118th Cong. (2023). All sources accessed online in September 2023.

Contact:
Scott Kraus, VP Sales & Marketing
Cardio Flow, Inc.
Mobile: (610) 247-3173
Email: skraus@cardioflow.net