February 2, 2021—Cardio Flow, Inc., announces completion of the enrollment phase of its FreedomFlow® Orbital…
December 7, 2017—Cardio Flow, Inc., announced today that it had received approval from the FDA of an Investigational Device Exemption (IDE) for its FreedomFlow® Orbital Atherectomy System First-in-Human (FIH) clinical trial. Cardio Flow will soon begin enrolling patients for this First-in-Human FAST Trial. The FDA approval was granted on on November 21, 2017.
Study Objective: To evaluate first-in-human safety and effectiveness of the Cardio Flow FreedomFlow® Orbital Atherectomy System for atherosclerotic plaque removal in de novo native target lesions in the peripheral vasculature of the lower extremities.
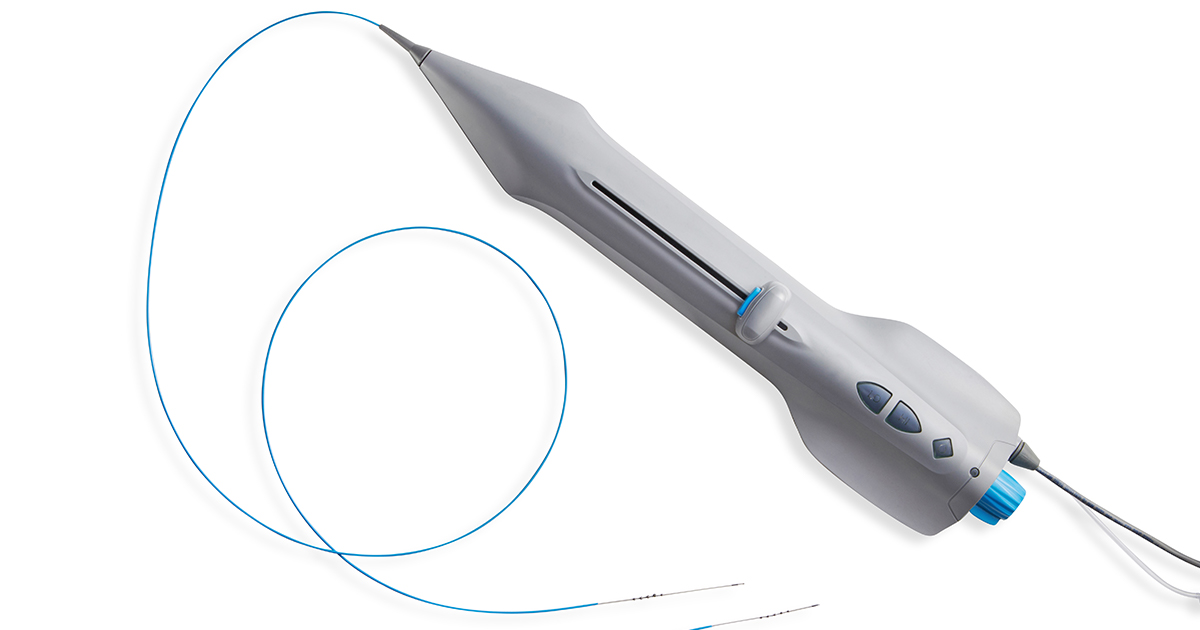
Investigational Product: Cardio Flow FreedomFlow® Orbital Atherectomy System.
- CM1001 – Control Module and Tubing Set
- H4001 – User Handle
Study Design: Prospective, 2 centers, non-randomized single-arm study.
Enrollment Size and Number of Sites: Up to 10 subjects at up to 2 sites in the United States.
Primary Effectiveness: Technical success, defined as the ability of the Cardio Flow FreedomFlow® Orbital Atherectomy System to achieve a residual diameter stenosis ≤50% post treatment with or without adjunctive therapy, as assessed by an independent Angiographic Core Laboratory.
Study Duration: Enrollment is expected to take approximately 2 months, with observation periods at time of procedure, pre-discharge, 30 days, and 6 months post-procedure follow-up.
Device indications for use: The FreedomFlow® Orbital Circumferential Atherectomy System is applied as therapy to remove atherosclerotic plaque within peripheral arterial vessels. The therapy is intended for patients who are acceptable candidates for percutaneous transluminal atherectomy.
CAUTION: Investigational device. Limited by United States law to investigational use.