PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release St. Paul, MN – October…
PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release
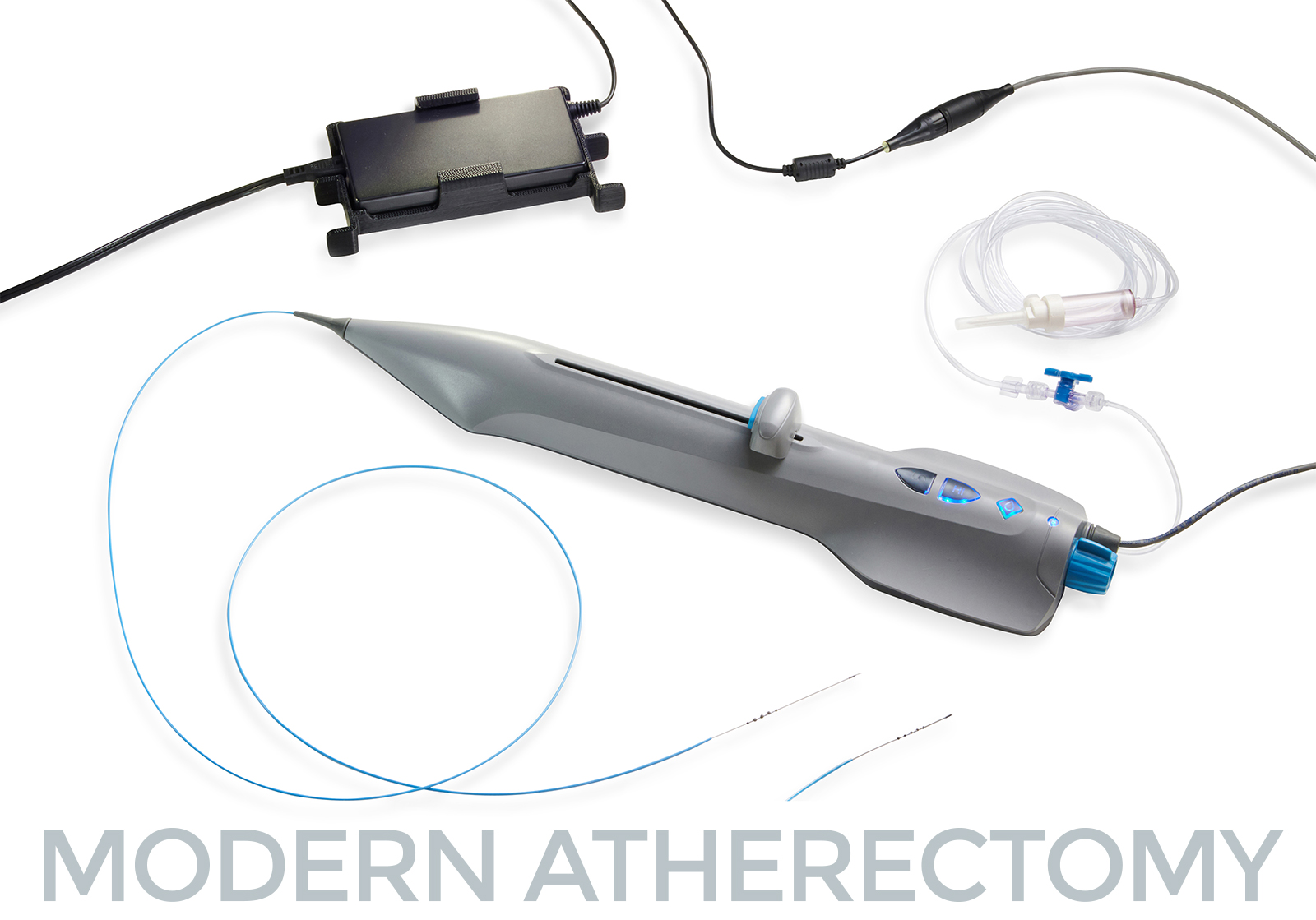
St. Paul, MN – October 30, 2023. Cardio Flow, Inc., a privately held commercial-stage medical device company and developer of minimally invasive devices to treat peripheral artery disease (PAD), is pleased to announce the successful completion of its first commercial cases for its FreedomFlow® Orbital Atherectomy Platform following FDA clearance in late September.
First Commercial Case Performed at the Cardiovascular Institute of the South (CIS)
Cardio Flow announced the successful completion of its first commercial cases in leading medical centers across the U.S. The initial cases were performed by Drs. Craig Walker, Pradeep Nair, and McCall Walker of the Cardiovascular Institute of the South (CIS) at the Terrebonne General Health System in Houma, LA.
FreedomFlow treats plaque blockages in the legs, and its unique technology gives physicians greater versatility in treating multiple arteries and multiple blockages in the same vessel—all with a single device. The first patient treated at Terrebonne had a complex arterial anatomy and multiple blockages with extensive calcium, including a chronic total occlusion. FreedomFlow’s dynamic new mechanism of action demonstrated its effectiveness in these initial procedures.
Dr. Pradeep Nair, M.D., an interventional cardiologist in Houma, LA, and a recognized leader in the treatment of PAD and critical limb-threatening ischemia, commented:
“Peripheral arterial blockages are extremely common, especially in our patients with heart disease. This new technology allows us to treat a wide range of blockages effectively from the ankle to the hip, with the goal of saving limbs—and ultimately our patients’ lives and the quality of those lives.”
FreedomFlow’s Innovative, Modern Approach to Atherectomy
FreedomFlow’s dynamic atherectomy platform is the latest technological advancement for treating patients with PAD and restoring blood flow. Its catheter-based design uses diamond-coated spheres to sand the blockage, leveraging the physics of angular momentum to keep the spheres in constant contact with the vessel wall, whether the device is advancing or retracting. A diamond-coated tip likewise helps physicians ease the driveshaft through tight blockages.
FreedomFlow’s modern mechanism of action maximizes operational efficiency and reduces procedural run times, and its simplicity offers time savings and cost efficiencies for physicians and clinics, with quick and simple device setup no capital equipment, no lubricant, and reduced inventory needs. It also provides consistent treatment outcomes and a nearly flat learning curve for physicians, making it an ideal choice for today’s hospitals, ambulatory surgical centers, and office-based labs.
Appointment of Chief Operating Officer
Michael Kallok, Ph.D., and CEO of Cardio Flow, also announced the appointment of Scott Kraus as Chief Operating Officer. Most recently, Scott served as Vice President of Sales & Marketing for Cardio Flow, and was responsible for overseeing all commercial functions. Previously, Scott worked for ABIOMED (acquired by JNJ), where he was a Zone General Manager, and before this worked in executive leadership roles at a series of start-ups: Cardiovascular Systems, Inc. (CSI, acquired by Abbott), Intact Vascular (acquired by Philips), and Intersect ENT (acquired by Medtronic).
Most notably, he was part of the commercial team at CSI that launched the orbital atherectomy systems. He subsequently became VP of Sales at CSI and led the commercial organization in generating $80 million in annual revenue.
Dr. Kallok stated:
“We are at a pivotal point at Cardio Flow, and expanding our leadership team in preparation for significant growth is paramount as we launch and scale the organization. We believe Scott has the operational and commercial expertise to improve organizational efficiencies and operational strategy to achieve our ambitious goals of delivering a portfolio of innovative endovascular solutions for PAD patients.”
About Cardio Flow, Inc.
Cardio Flow, Inc., is a privately held medical device company located in St. Paul, MN, which designs and develops minimally invasive peripheral vascular products with the goal of providing physicians with better treatment options for peripheral vascular disease to improve patients’ lives.
See cardioflow.net for more information.
About Cardiovascular Institute of the South
The Cardiovascular Institute of the South (CIS) is a world leader in preventing, detecting, and treating cardiovascular and peripheral vascular disease, and has earned international acclaim as a pioneer of research, development, and education, as well as an innovator in the treatment of peripheral artery disease.
With a dedicated team of more than 1,075 members, CIS provides comprehensive cardiovascular care at 21 locations across Louisiana and Mississippi.
See cardio.com for more information.
Contact:
Scott Kraus, Chief Operating Officer
Cardio Flow, Inc.
Mobile: (610) 247-3173
Email: skraus@cardioflow.net
Mailing address
Cardio Flow, Inc.
525 Main Street, Box 120018
St. Paul, MN 55112 USA
Physical address
Cardio Flow, Inc.
3530 88th Ave NE
Blaine, MN 55014 USA