News
Cardio Flow Announces Completion of First Commercial Cases—and Appointment of Chief Operating Officer
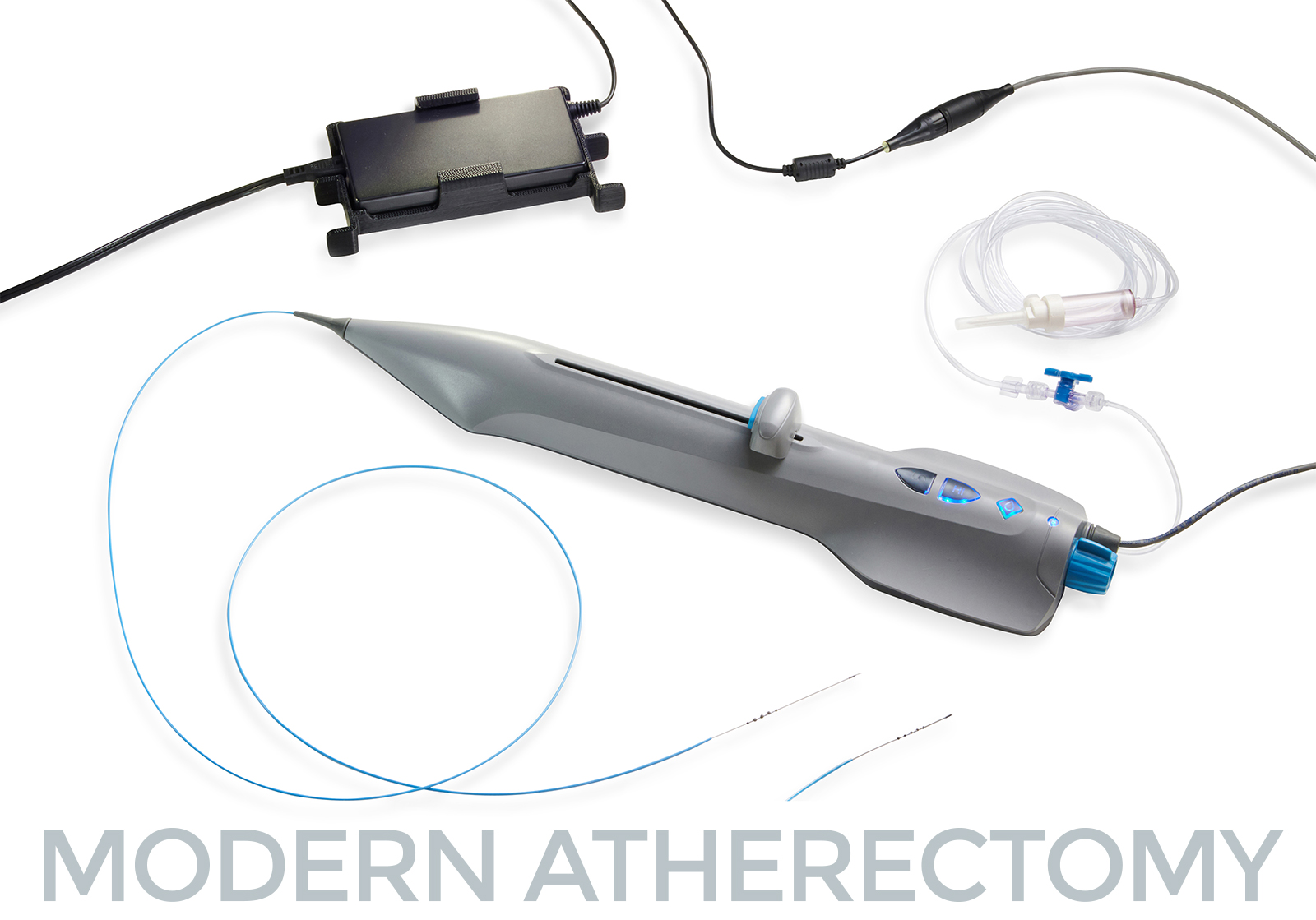
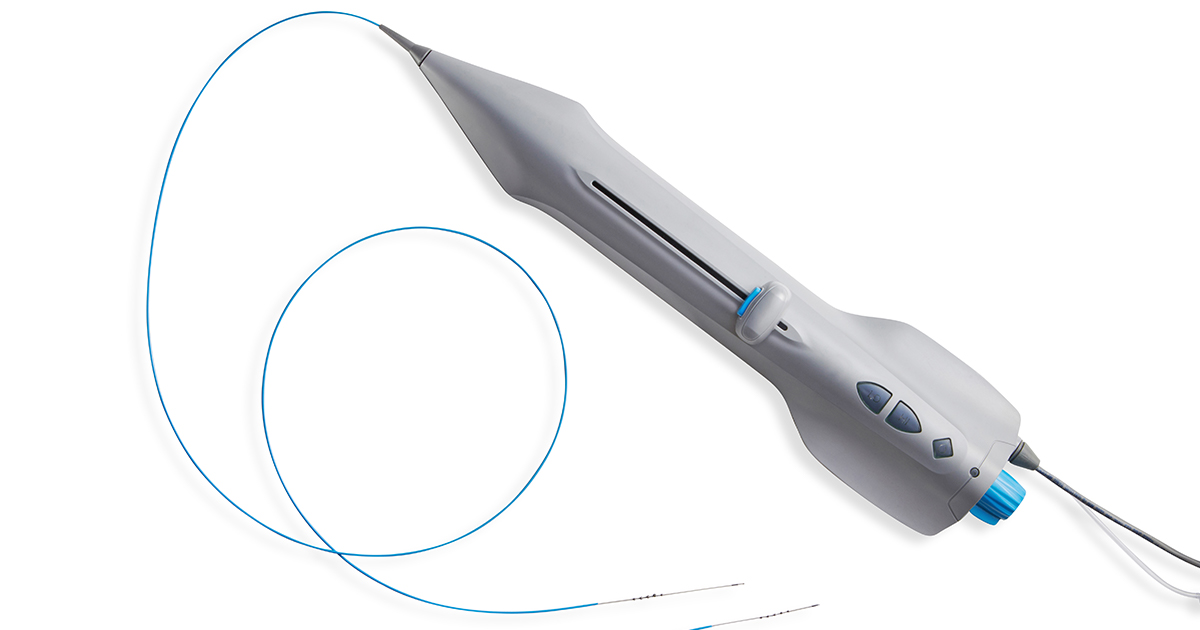
PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release St. Paul, MN – October 30, 2023. Cardio Flow, Inc., a privately held commercial-stage medical device company and developer of minimally invasive devices to treat peripheral artery disease (PAD), is pleased to announce the successful completion of its first commercial cases for its FreedomFlow® Orbital Atherectomy Platform following FDA clearance…
FreedomFlow Orbital Atherectomy Platform Receives FDA 510(k) Clearance to Treat Peripheral Artery Disease
PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release St. Paul, MN – October 18, 2023. Cardio Flow, Inc., a privately held medical device company and developer of minimally invasive devices to treat peripheral artery disease (PAD), today announced it recently received U.S. Food and Drug Administration (FDA) 510(k) clearance for the company’s FreedomFlow Orbital Atherectomy Peripheral Platform.
Cardio Flow, Inc., Announces FDA Clearance for FreedomFlow Peripheral Guidewire, plus First Commercial Case Completed in U.S.
PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release St. Paul, MN – June 2, 2022 – Cardio Flow, Inc., a medical device company and developer of minimally invasive peripheral vascular devices to treat peripheral artery disease (PAD), today announced it recently received U.S. Food and Drug Administration (FDA) clearance for the company’s FreedomFlow® Peripheral Guidewire.
Cardio Flow to Present FAST II 30-Day Follow-Up Results at AMP Symposium in Orlando, August 11-14, 2021
July 23, 2021—Cardio Flow, Inc., will be attending the Amputation Prevention Symposium (AMP) in Orlando, Florida, August 11-14, 2021, and will be presenting the results of its 30-day follow-up study of the FAST II clinical trial of its FreedomFlow® orbital circumferential atherectomy system. Cardio Flow completed the enrollment phase of the FAST II clinical study, “Evaluation of the Cardio Flow FreedomFlow™…
Cardio Flow Announces Sales and Marketing Team
July 15, 2021—Cardio Flow, Inc., announces that the following individuals have recently accepted positions in its newly established Sales & Marketing team:
Cardio Flow completes enrollment within its FAST II Clinical Trial of the FreedomFlow Orbital Atherectomy System
February 2, 2021—Cardio Flow, Inc., announces completion of the enrollment phase of its FreedomFlow® Orbital Atherectomy device within its FAST II clinical trial, “Evaluation of the Cardio Flow FreedomFlow™ Orbital Circumferential Atherectomy System to Treat Peripheral Artery Disease.” The study is evaluating the safety and effectiveness of the Cardio Flow FreedomFlow Orbital Circumferential Atherectomy System for atherosclerotic plaque removal and…
Cardio Flow Receives FDA IDE Approval for an Electrically Powered Variation of the FreedomFlow Orbital Atherectomy System
September 30, 2020—Cardio Flow, Inc., announced today that it has received approval by the U.S. Food and Drug Administration (FDA) for the addition of an electrically powered variation of its FreedomFlow® Orbital Atherectomy device to its investigational plan for its FAST II Trial, “Evaluation of the Cardio Flow FreedomFlow® Orbital Circumferential Atherectomy System to Treat Peripheral Artery Disease”. The electrically…
Cardio Flow Announces its FAST II Trial to Evaluate the Safety and Efficacy of the FreedomFlow™ Orbital Atherectomy System to Treat Peripheral Artery Disease (FAST II)
August 17, 2018. Cardio Flow announced the start of its FAST II trial to evaluate the safety and effectiveness of its FreedomFlow™ Orbital Circumferential Atherectomy System for atherosclerotic plaque removal in subjects diagnosed with peripheral arterial disease of the lower extremities. Cardio Flow will begin enrolling patients for this study in late 2018. Study Objective: To evaluate the safety and…
Cardio Flow Announces its FAST II Trial to Evaluate the Safety and Efficacy of the FreedomFlow Orbital Atherectomy System to Treat Peripheral Artery Disease (FAST II)
August 17, 2018—Cardio Flow, Inc., announced today the start of its FAST II trial to evaluate the safety and effectiveness of its FreedomFlow® Orbital Atherectomy System for atherosclerotic plaque removal in subjects diagnosed with peripheral arterial disease of the lower extremities. Cardio Flow will begin enrolling patients for this study in late 2018. Study Objective: To evaluate the safety and…
Cardio Flow Announces Feasibility Clinical Trial of the Cardio Flow FreedomFlow Orbital Atherectomy System to Treat Peripheral Artery Disease (FAST Trial)
December 7, 2017—Cardio Flow, Inc., announced today that it had received approval from the FDA of an Investigational Device Exemption (IDE) for its FreedomFlow® Orbital Atherectomy System First-in-Human (FIH) clinical trial. Cardio Flow will soon begin enrolling patients for this First-in-Human FAST Trial. The FDA approval was granted on on November 21, 2017. Study Objective: To evaluate first-in-human safety and effectiveness of…