February 2, 2021—Cardio Flow, Inc., announces completion of the enrollment phase of its FreedomFlow® Orbital…
July 23, 2021—Cardio Flow, Inc., will be attending the Amputation Prevention Symposium (AMP) in Orlando, Florida, August 11-14, 2021, and will be presenting the results of its 30-day follow-up study of the FAST II clinical trial of its FreedomFlow® orbital circumferential atherectomy system.
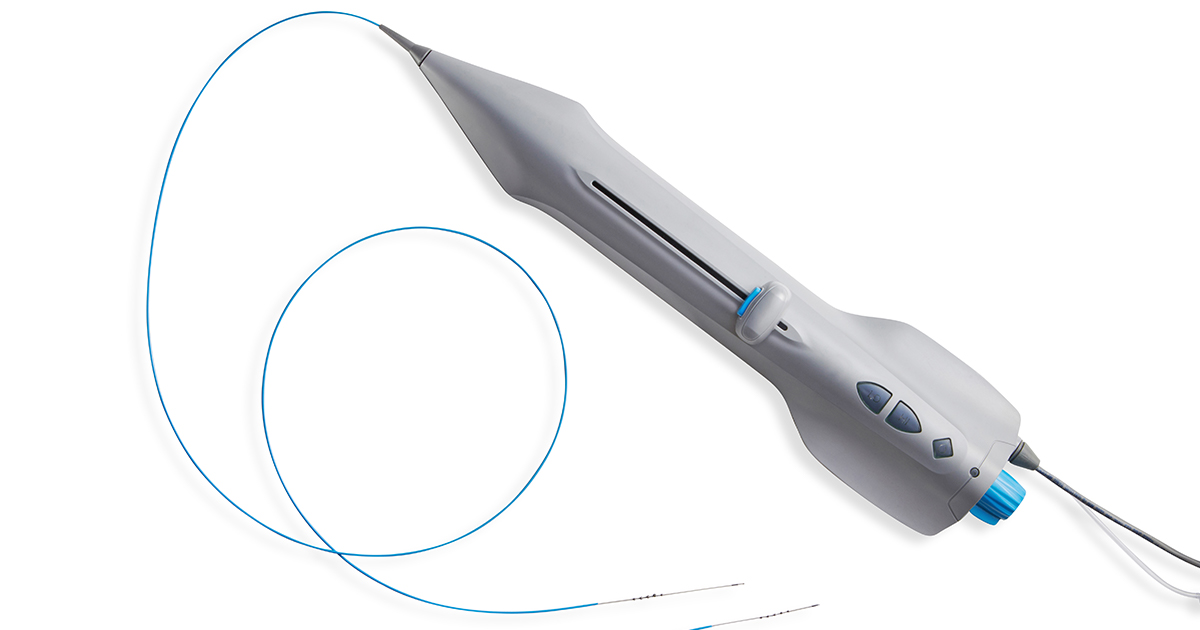
Cardio Flow completed the enrollment phase of the FAST II clinical study, “Evaluation of the Cardio Flow FreedomFlow™ Orbital Circumferential Atherectomy System to Treat Peripheral Artery Disease,” in February.
Thomas Davis, M.D., co-principal investigator for the FAST II study, will present the FreedomFlow system and 30-day follow-up clinical data on Thursday, August 12 at 9:10 AM in the Regency Ballroom at the Hyatt Regency Orlando. Dr. Davis is an interventional cardiologist with St. John Hospital and Medical Center in St. Clair Shores, Michigan. Cardio Flow will also have a booth in “Innovation Row” at Table 118.
Cardio Flow contact for the AMP Symposium: Scott Kraus, Vice President of Sales & Marketing – e-mail or call (610) 247-3173.
Virtual passes are available for the AMP Symposium here.
The FAST II study evaluated the safety and effectiveness of the Cardio Flow FreedomFlow® Orbital Circumferential Atherectomy System for atherosclerotic plaque removal and vessel compliance modification in de novo native target lesions in the peripheral vasculature of the lower extremities. Enrollment involved a total of 112 patients at 13 locations with 25 physicians in the United States.
Device indications for use: The FreedomFlow™ Orbital Circumferential Atherectomy System is applied as therapy to remove atherosclerotic plaque within peripheral arterial vessels. The therapy is intended for patients who are acceptable candidates for percutaneous transluminal atherectomy.
CAUTION: Investigational device. Limited by United States law to investigational use.