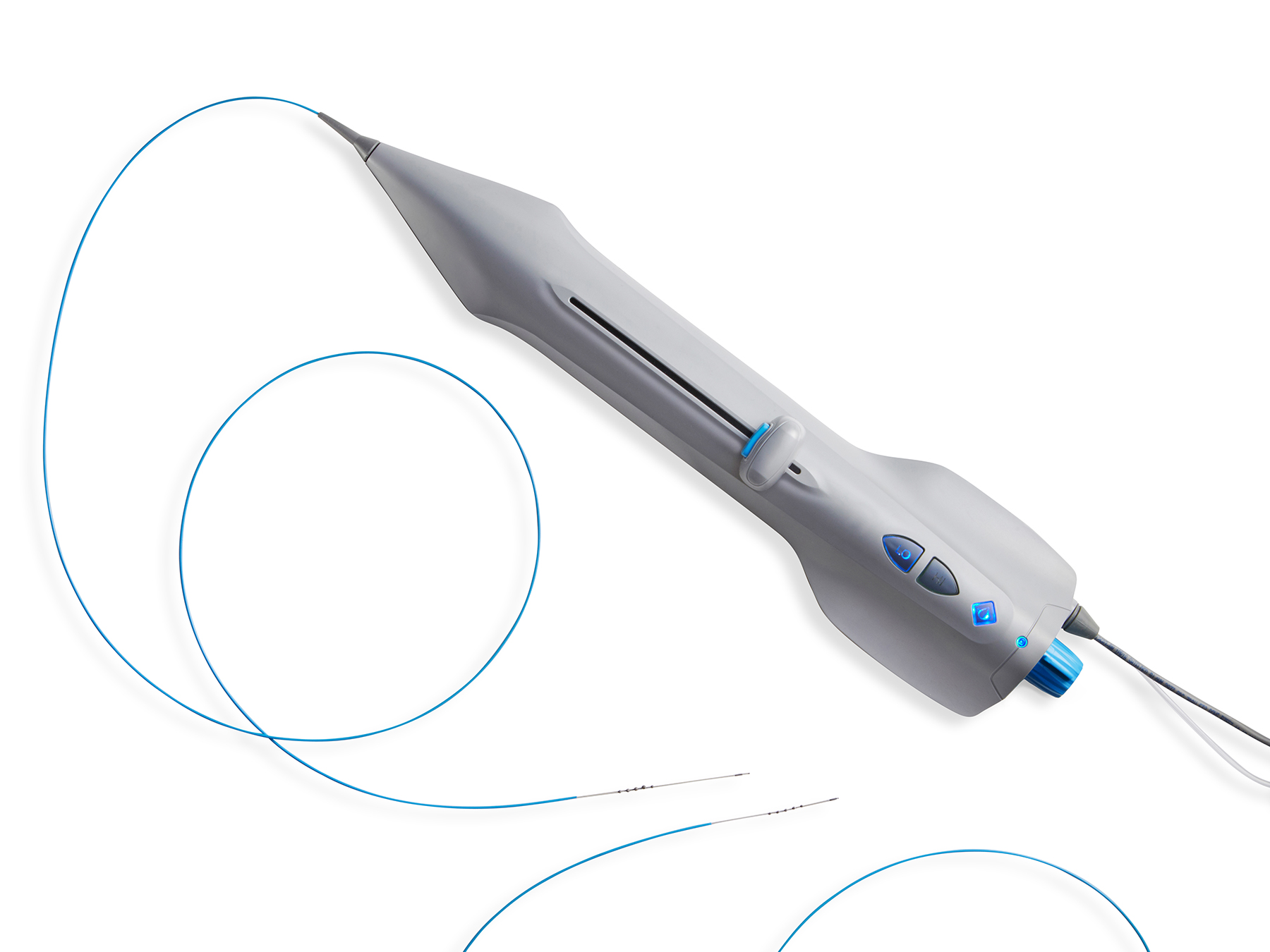
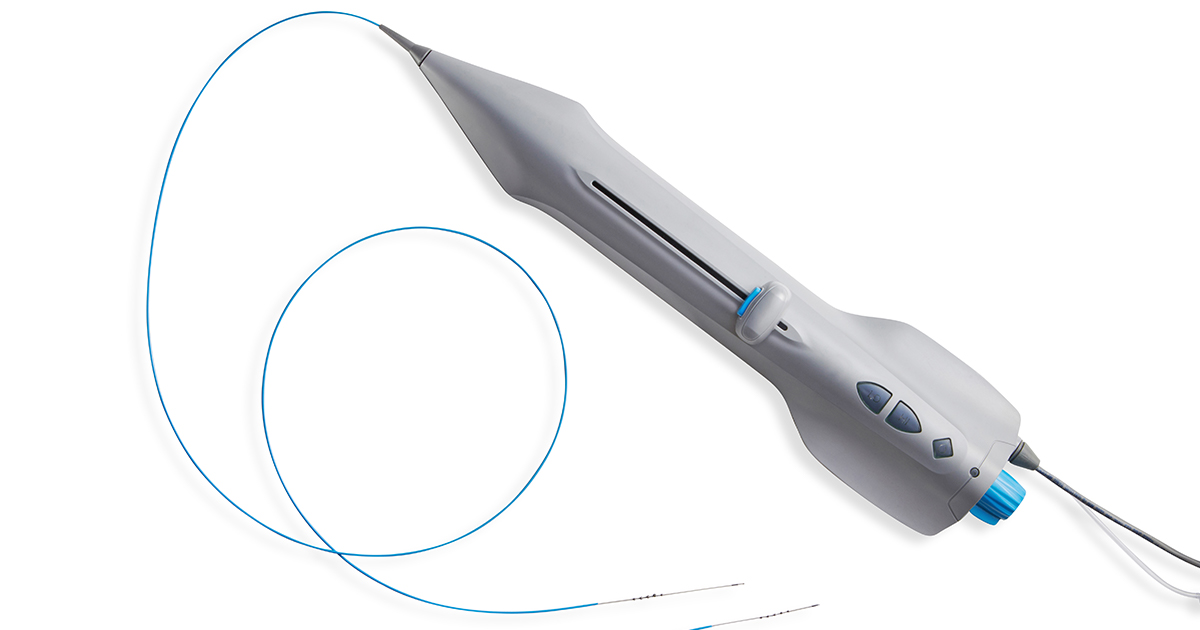
February 2, 2021—Cardio Flow, Inc., announces completion of the enrollment phase of its FreedomFlow® Orbital…
September 30, 2020—Cardio Flow, Inc., announced today that it has received approval by the U.S. Food and Drug Administration (FDA) for the addition of an electrically powered variation of its FreedomFlow® Orbital Atherectomy device to its investigational plan for its FAST II Trial, “Evaluation of the Cardio Flow FreedomFlow® Orbital Circumferential Atherectomy System to Treat Peripheral Artery Disease”.
The electrically powered user handle will be added to the study, which includes the previously approved pneumatically powered user handle.
The new user handle features an integrated electric motor and a nonsterile, reusable power supply. The approval was granted by the FDA on September 25, 2020.
Device indications for use: The FreedomFlow® Orbital Circumferential Atherectomy System is applied as therapy to remove atherosclerotic plaque within peripheral arterial vessels. The therapy is intended for patients who are acceptable candidates for percutaneous transluminal atherectomy.
CAUTION: Investigational device. Limited by United States law to investigational use.