July 23, 2021—Cardio Flow, Inc., will be attending the Amputation Prevention Symposium (AMP) in Orlando,…
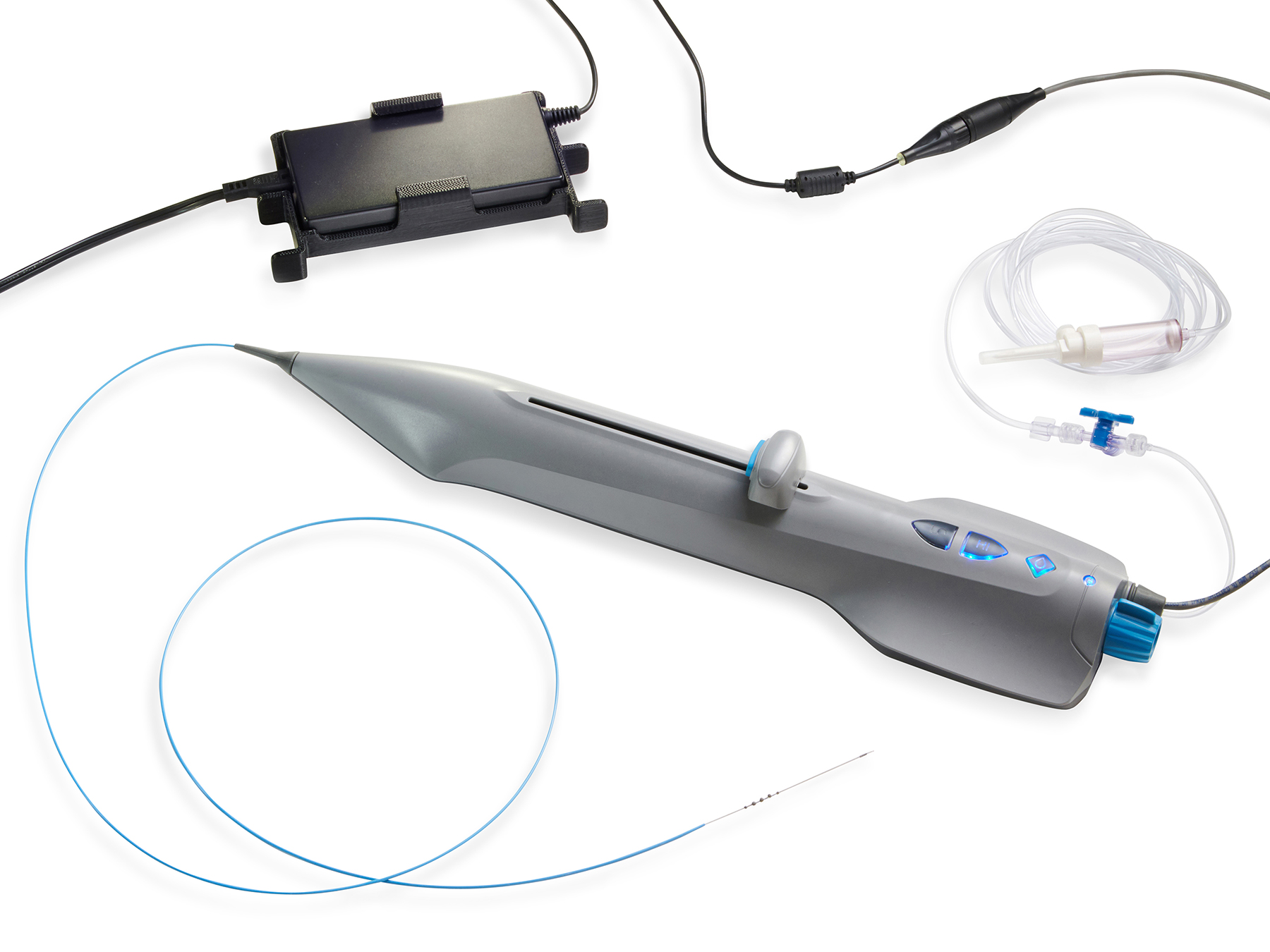
February 2, 2021—Cardio Flow, Inc., announces completion of the enrollment phase of its FreedomFlow® Orbital Atherectomy device within its FAST II clinical trial, “Evaluation of the Cardio Flow FreedomFlow™ Orbital Circumferential Atherectomy System to Treat Peripheral Artery Disease.”
The study is evaluating the safety and effectiveness of the Cardio Flow FreedomFlow Orbital Circumferential Atherectomy System for atherosclerotic plaque removal and vessel compliance modification in de novo native target lesions in the peripheral vasculature of the lower extremities.
The study enrolled a total of 112 patients at 13 locations with 25 physicians in the United States, and evaluated both an electrically powered and a pneumatically powered user handle.
Device indications for use: The FreedomFlow® Orbital Circumferential Atherectomy System is applied as therapy to remove atherosclerotic plaque within peripheral arterial vessels. The therapy is intended for patients who are acceptable candidates for percutaneous transluminal atherectomy.
CAUTION: Investigational device. Limited by United States law to investigational use.