The FreedomFlow Platform
Orbital Atherectomy Platform for the Treatment of Peripheral Artery Disease
Catheter, Driveshaft, Tubing Set, and Power Supply
The FreedomFlow® platform is a minimally invasive, catheter-based endovascular device for removing atherosclerotic plaque from peripheral arterial blood vessels within the body.
Instructions for Use (IFU)
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Platform Components
Catheter
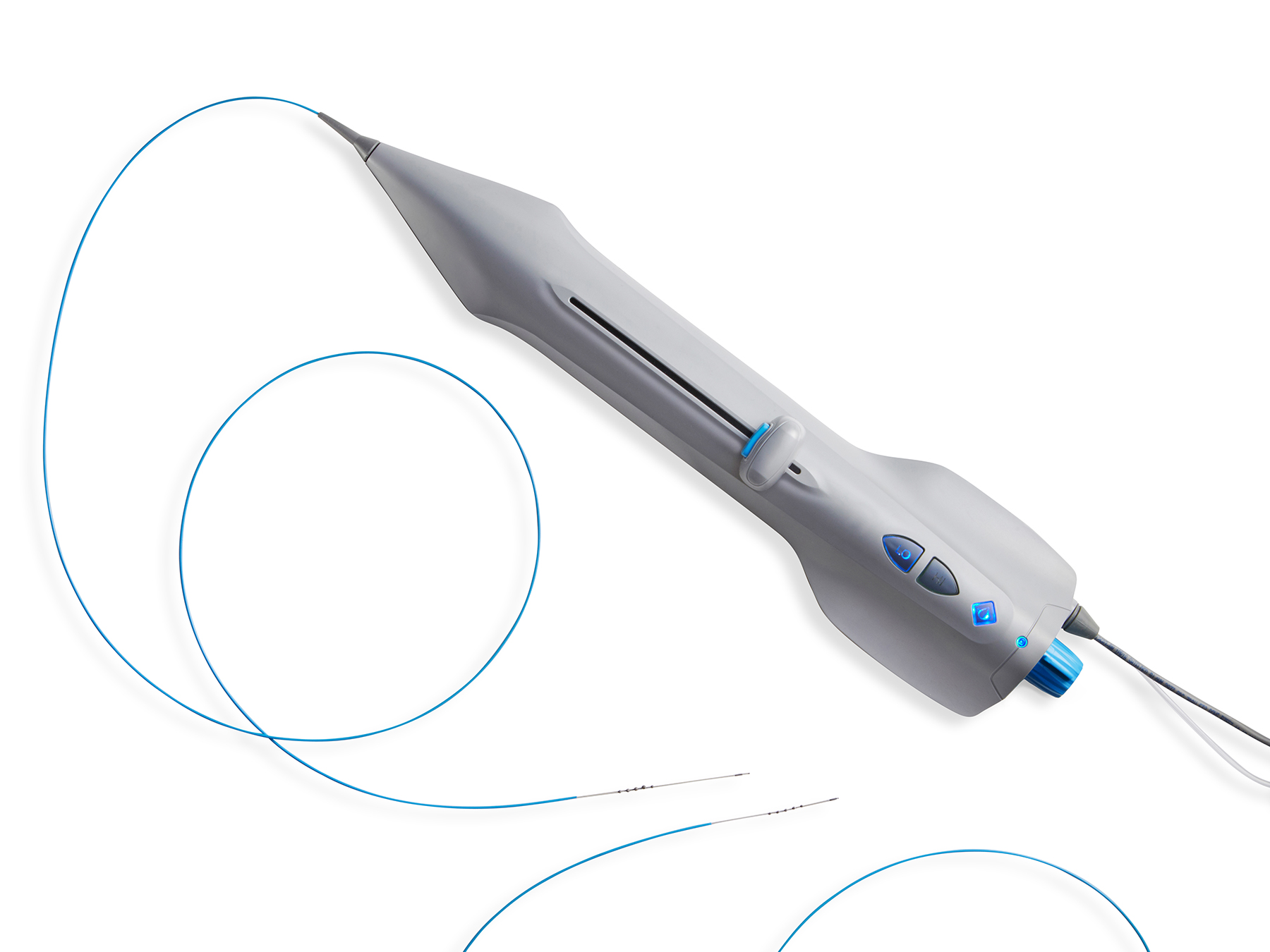
Controls rotational and translational movement of the driveshaft
The single-use catheter with integrated electric motor and saline pump provides control over the rotational and translational movement of the integrated driveshaft.
The blue button on the catheter activates the rotation of the driveshaft when depressed; rotation stops when the button is released.
Two catheter options are available to treat a wide range of vessel sizes— from 2 mm to 8 mm:
Driveshaft

Multi-strand cable drives the rotation of the diamond coated spheres
The driveshaft is a hollow, multi-strand cable that drives the rotation of the diamond-coated spheres. The off-axis eccentric attachment of the spheres onto the driveshaft creates an outward angular momentum during rotation due to centrifugal force.
The diamond-coated hypotube is designed to ease the tip through tight blockages.
This driveshaft is part of the catheters H6001 and H6002.
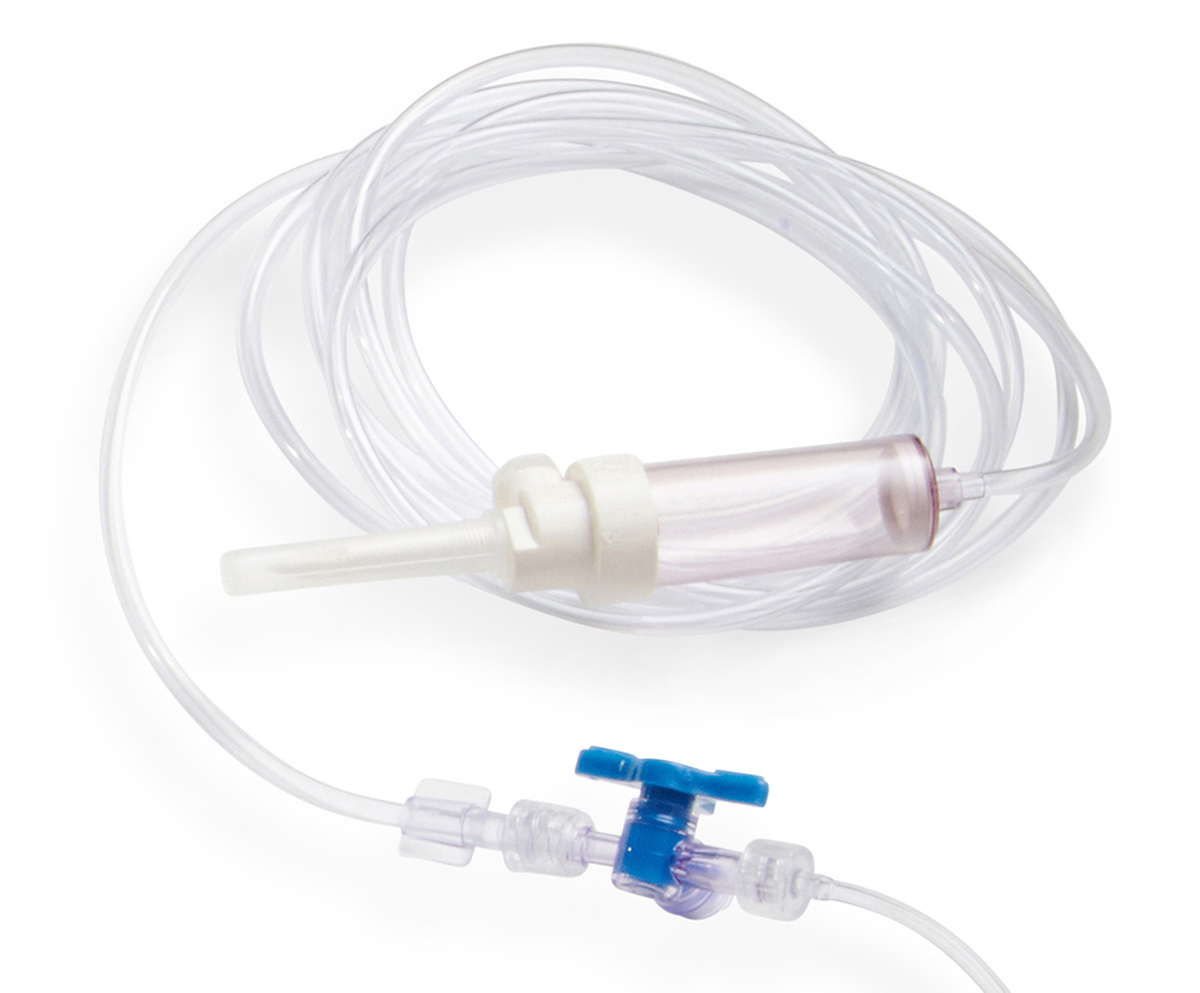
Tubing set
Delivers saline to the catheter
The tubing set acts as a conduit for standard saline solution to the saline pump, which is integrated into the catheter.
This item is provided sterile with the packaged catheters H6001 and H6002.
Power supply
Delivers electric power to the catheter
The power supply is a reusable, non-sterile AC-to-DC Medical Grade Class II power converter with bracket for attaching to a standard hospital bed.
Model number H7001
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
INDICATIONS FOR USE: The Cardio Flow FreedomFlow® Orbital Atherectomy Peripheral Platform is indicated for use as therapy in patients with occlusive atherosclerotic disease in peripheral arteries. The therapy is intended for patients who are acceptable candidates for percutaneous transluminal atherectomy.