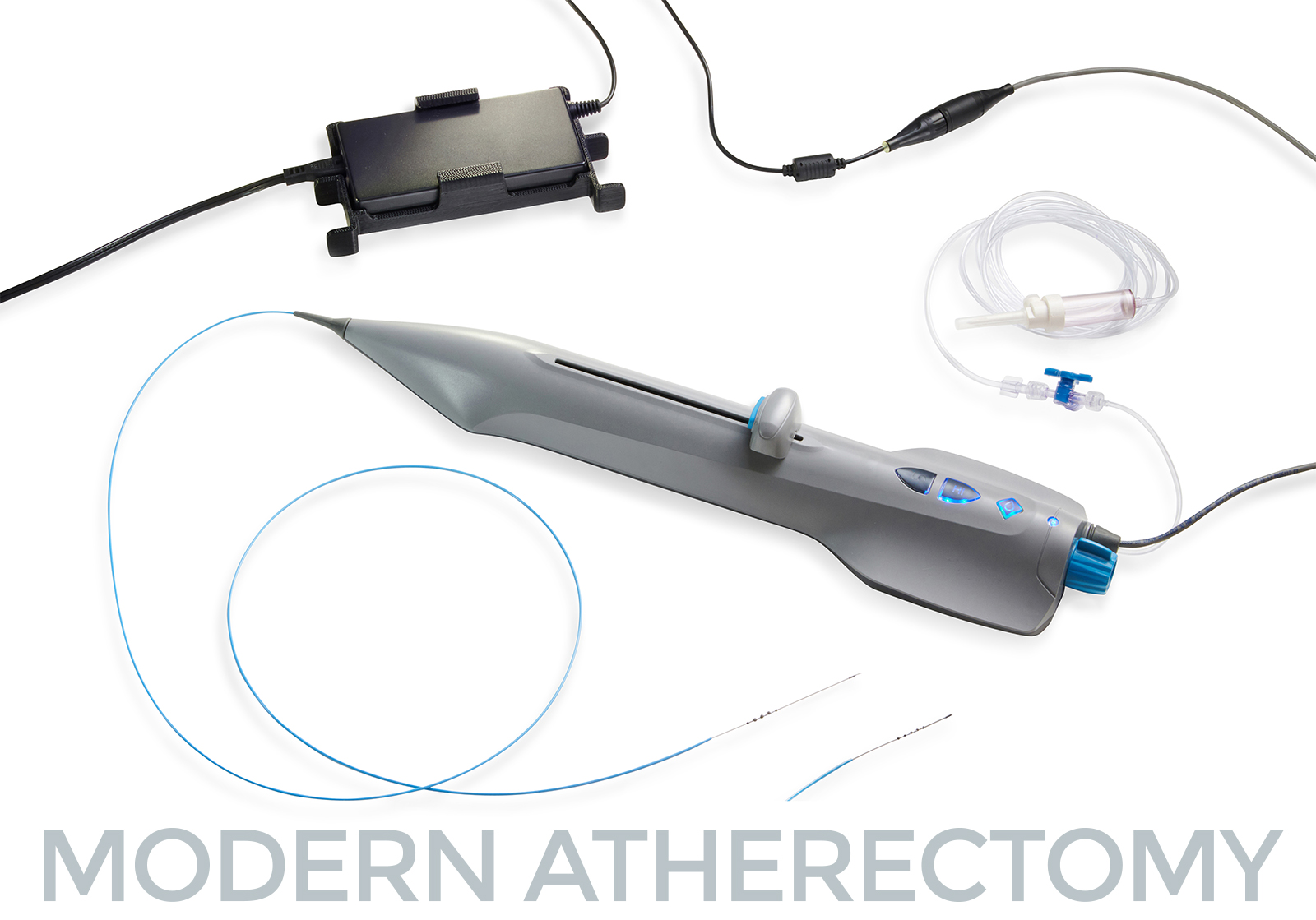
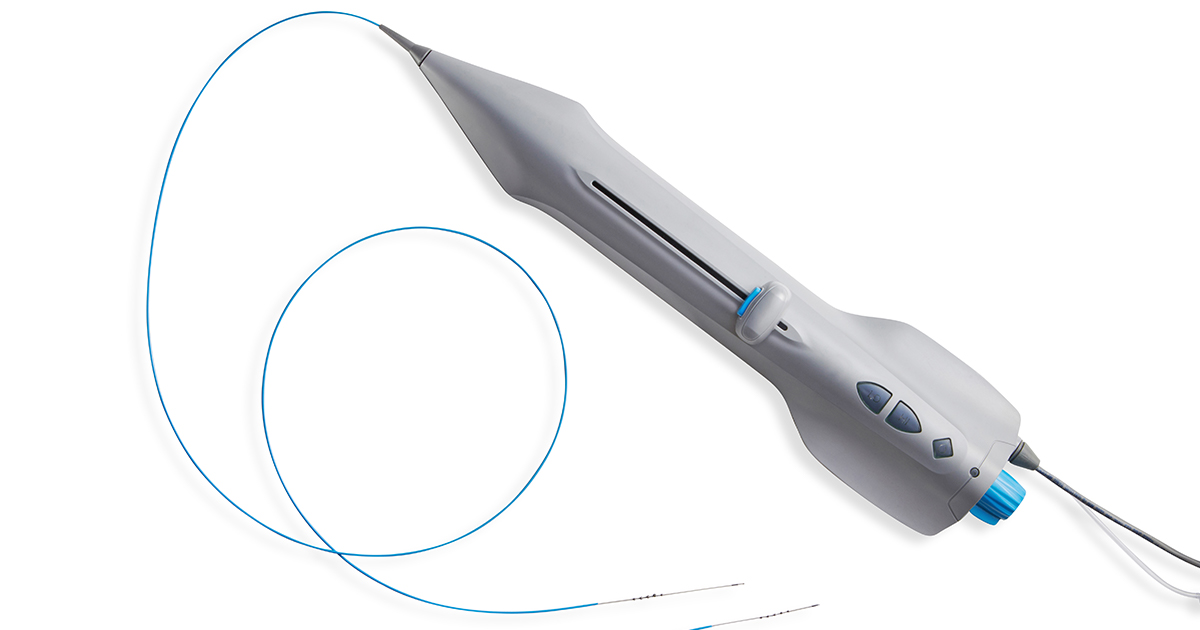
Cardio Flow Announces Completion of First Commercial Cases—and Appointment of Chief Operating Officer
PRESS RELEASE | FOR IMMEDIATE RELEASE | Download press release St. Paul, MN – October 30, 2023. Cardio Flow, Inc., a privately held commercial-stage medical device company and developer of minimally invasive devices to treat…