Cardio Flow FreedomFlow Peripheral Guidewire
Designed to cross and treat lesions both above and below the knee
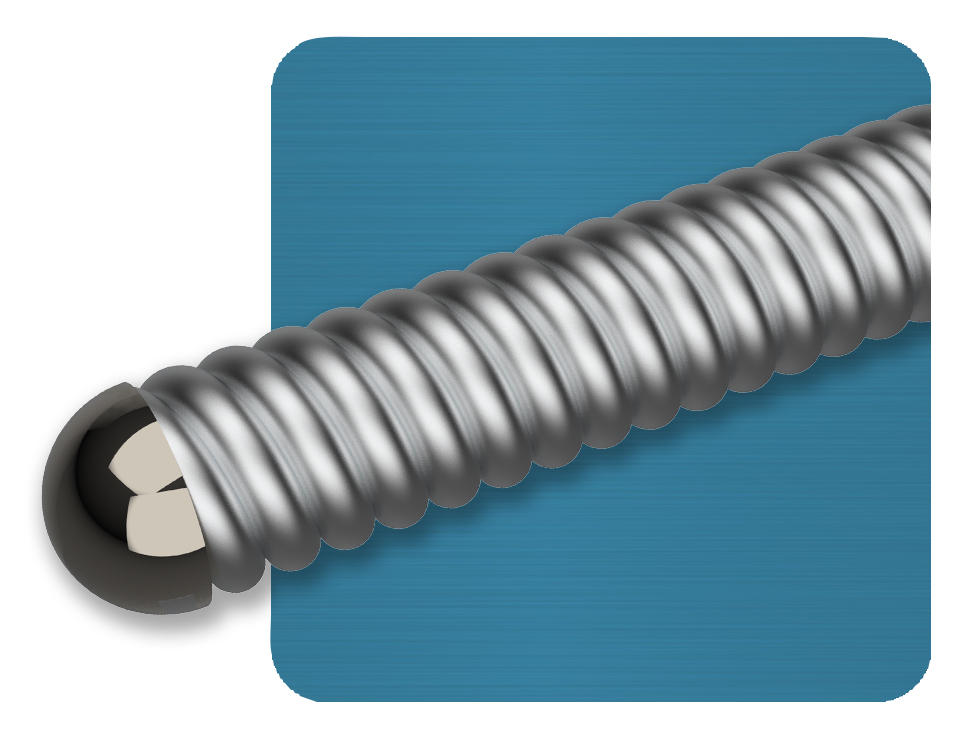
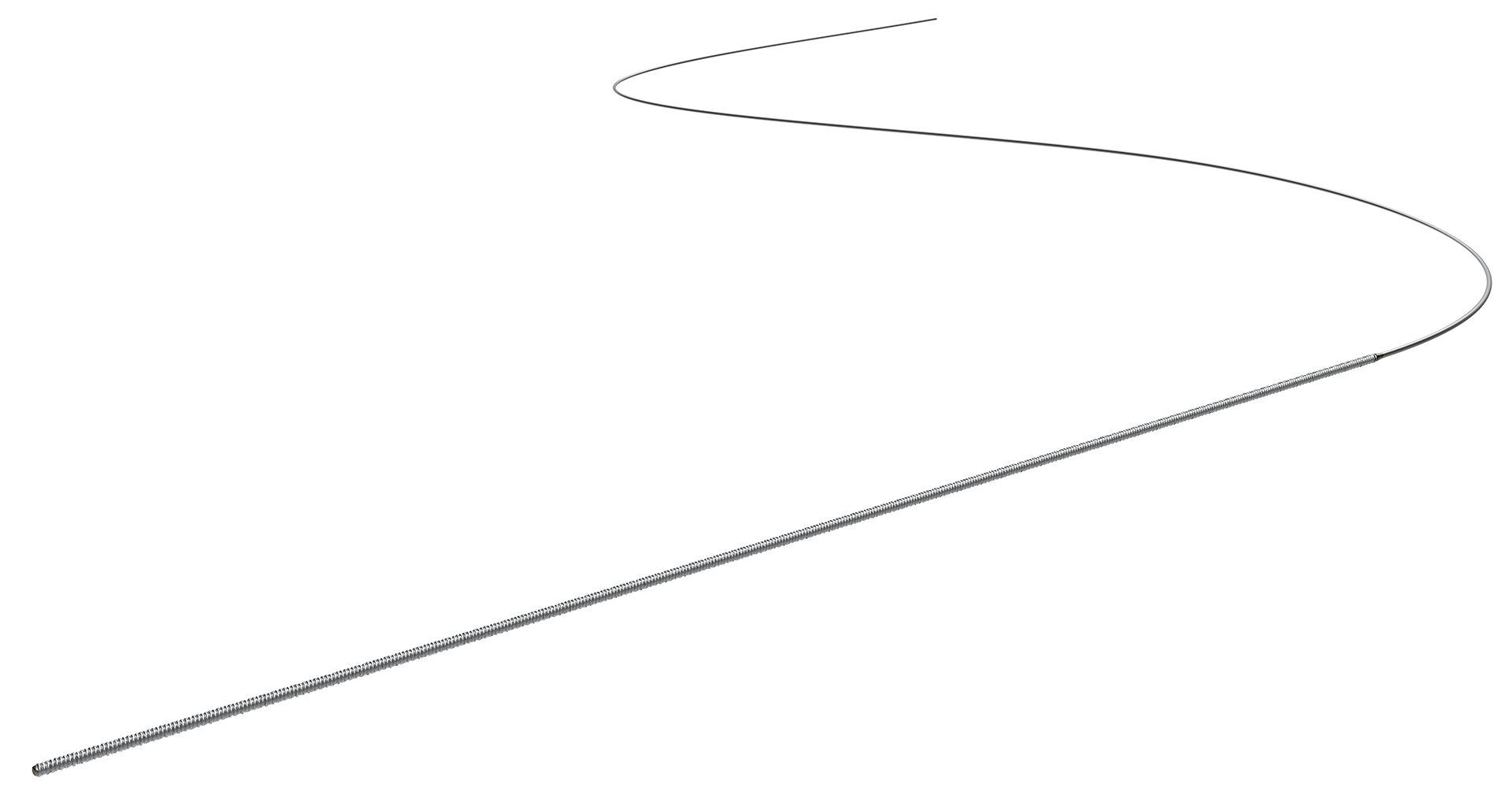
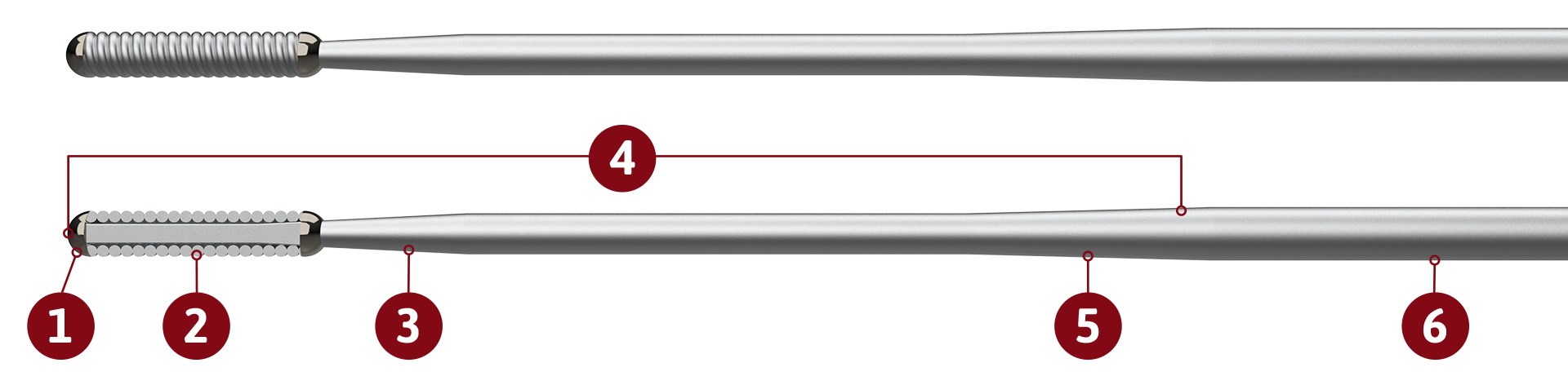
The FreedomFlow Peripheral Guidewire is a stainless-steel guidewire with a fixed distal-spring coil and is designed to deliver exceptional support for therapeutic devices.
Features
Deliverability
Fixed distal silicone-coated spring coil eases the crossing of difficult lesions.
Control
Core-to-tip design and medium-stiff coil facilitates superior torque transmission and control.
Support
Stainless-steel mandrel (0.014 inch) is designed to provide excellent support.
Cardio Flow FreedomFlow Peripheral Guidewire GW1001 Specifications
Model
GW1001
Nominal Core Outer Diameter
0.013 inch (0.33 mm)
Maximum Core Outer Diameter
0.014 inch (0.36 mm)
Maximum Spring Tip Outer Diameter
0.0144 inch (0.366 mm)
Recommended Introducer Sheath
0.014 inch minimum (0.36 mm) lumen compatibility
Length
325 cm
Tip Load Force
13 gf (Reference)
To order
Please contact Cardio Flow at 800-294-5517 to order the FreedomFlow Peripheral Guidewire.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
INDICATIONS FOR USE: The Cardio Flow FreedomFlow Peripheral Guidewire is intended for temporary placement in peripheral vasculature to facilitate the placement and exchange of diagnostic and therapeutic devices during percutaneous intravascular procedures. This guidewire device is intended for peripheral vascular use only.
Refer to the Instructions for Use for a complete listing of the indications, contraindications, warnings, and precautions.